Databases
AddictedChem is the firstly comprehensive knowledge base of controlled substances and a platform for predicting NPS. AddictedChem will help the experiment accelerate the research of controlled substances and assist the rapid detection of NPS.
1. Flow chart

Figure 1. Flow chart of AddictedChem. On the left is a multi-level database of controlled substances, and on the right is a prediction platform for NPS.
2. Statistics
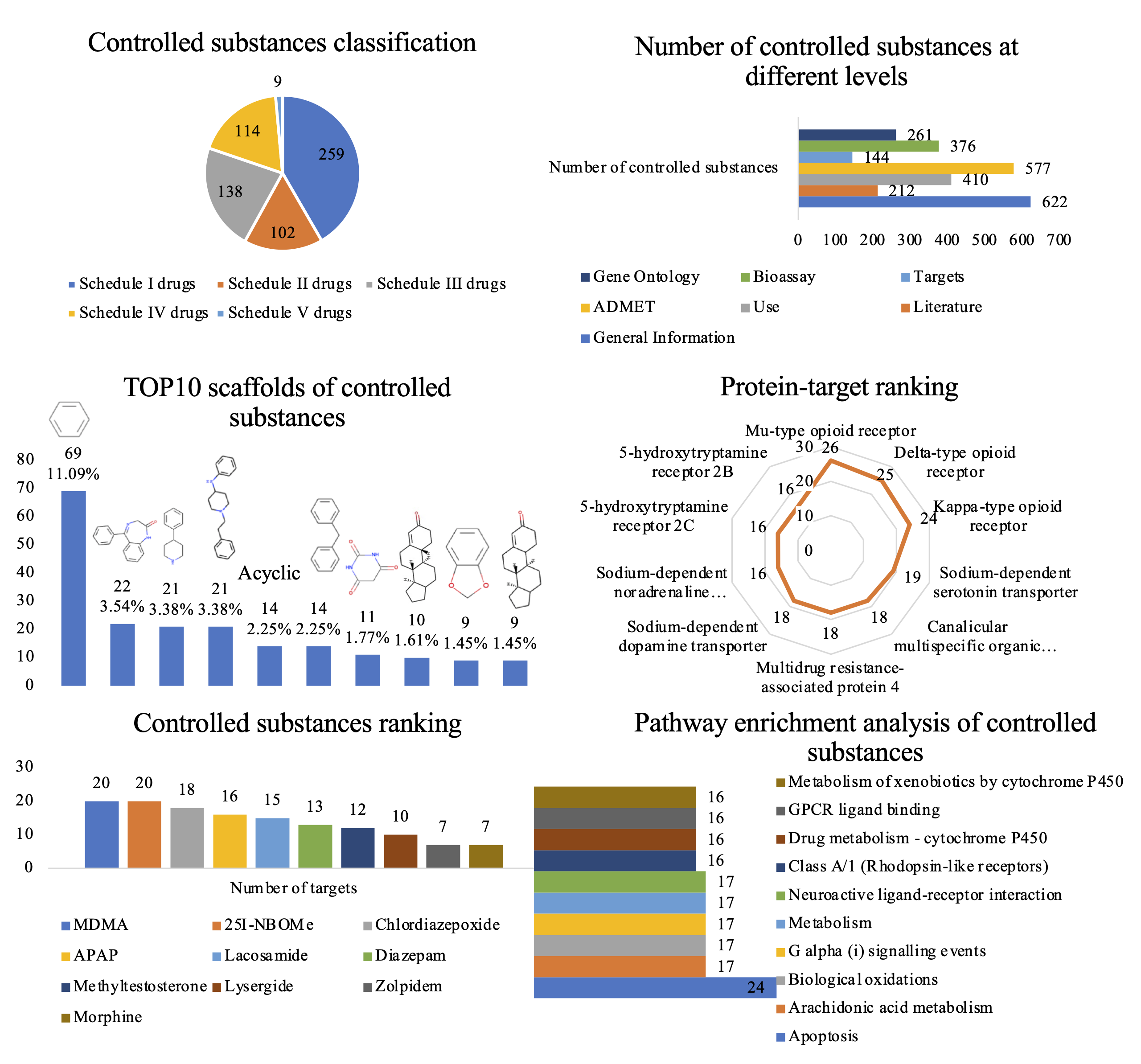
Figure 2. Statistical analysis of the entries in AddictedChem. (A) Classification of controlled substances. (B) Number of controlled substances at different levels. (C) TOP10 scaffolds of controlled substances. (D) Protein targets rankings according to the number of controlled substances. (E) Rankings of controlled substances according to the number of targets. (F) Pathway enrichment analysis of controlled substances.
1. Text query

Figure 3. Users can directly enter the name or structure of the controlled substances to obtain relevant literature, targets, ADMET, genes, pathways, use information and bioassay information of controlled substances.
2. Structure
Figure 4. The structure search method requires the user to provide the SMILES format of the compound or use the JSME tool to draw the structure of the compound.
3. Similiarity
Figure 5. The similarity retrieval method is based on the similarity algorithm, it will return the 20 closest molecular structures of the current compound in the AddictedChem database.
4. MCS
Figure 6. The MCS retrieval method is based on the fMCS algorithm, it will return to the 20 compounds with the largest substructure of the current compound in the AddictedChem database.
5. Fragment
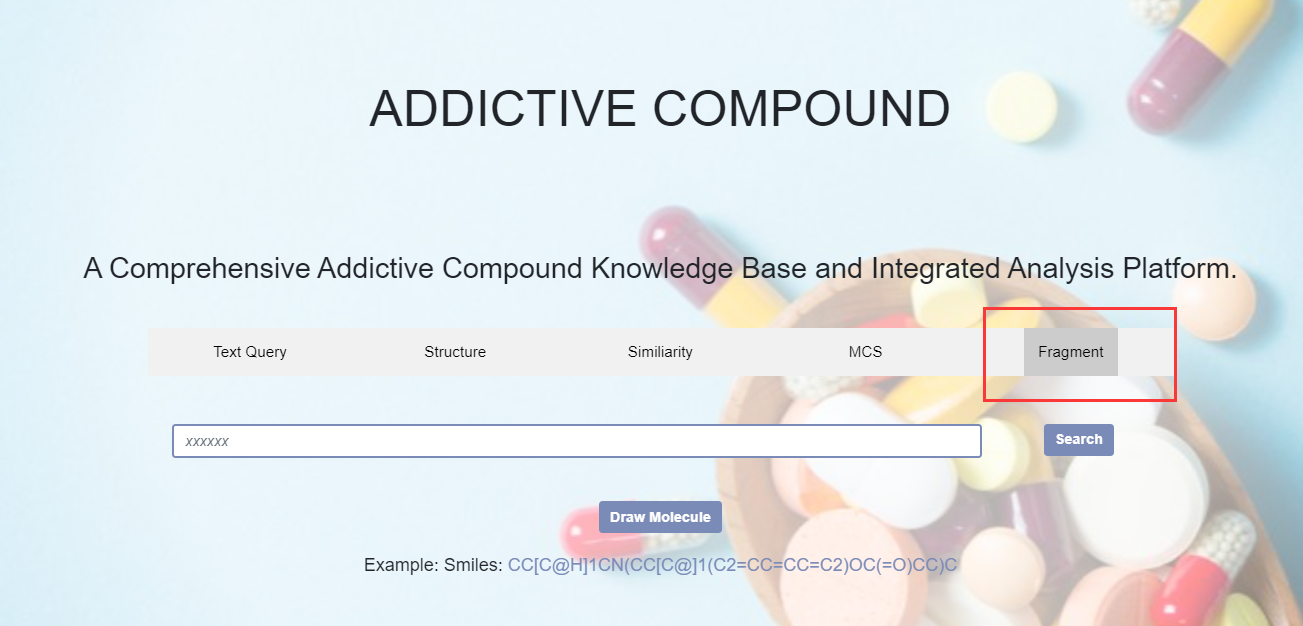
Figure 7. The fragment retrieval method is based on the theory of connected subgraphs. After entering the chemical structure of a compound, all fragment structures of the compound will be obtained.
Prediction
1. Prediction Entry
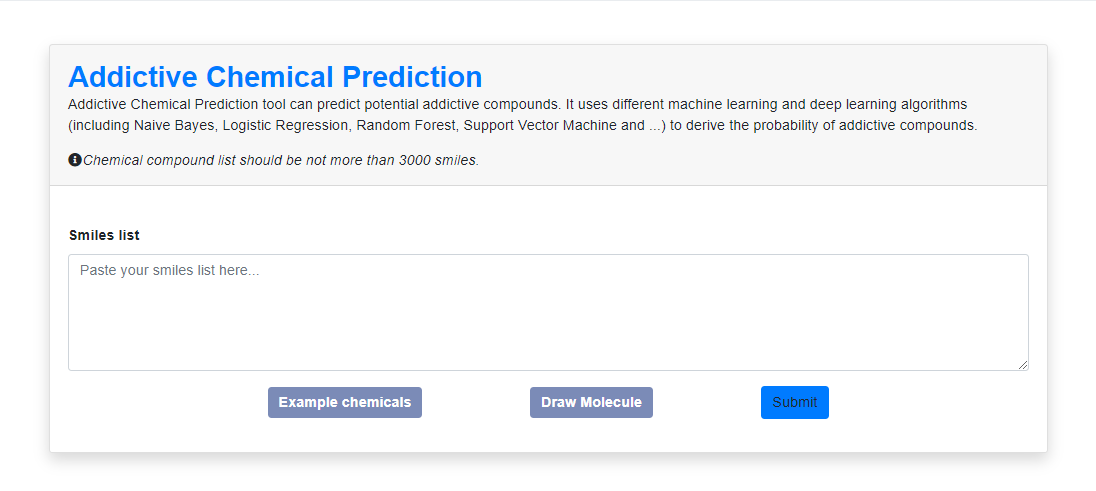
Figure 8. The entry of prediction. In the prediction platform part, the users can get the result reports by entering the SMILES form of the compound or some compounds divided by rows.
2. Prediction Results
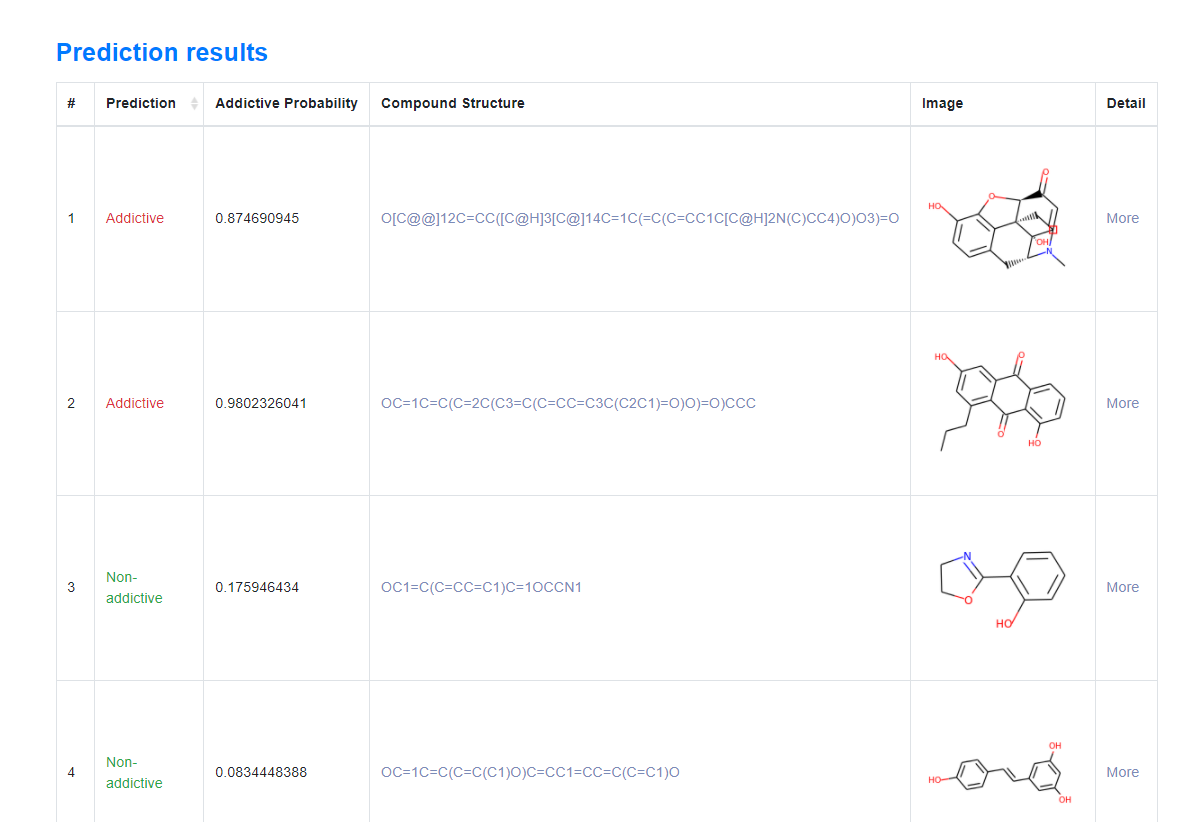
Figure 9. After the user enters the smiles of multiple compound molecules, multiple results will be displayed in the form of a list. Click More to view the details.
3. Prediction Results Detail
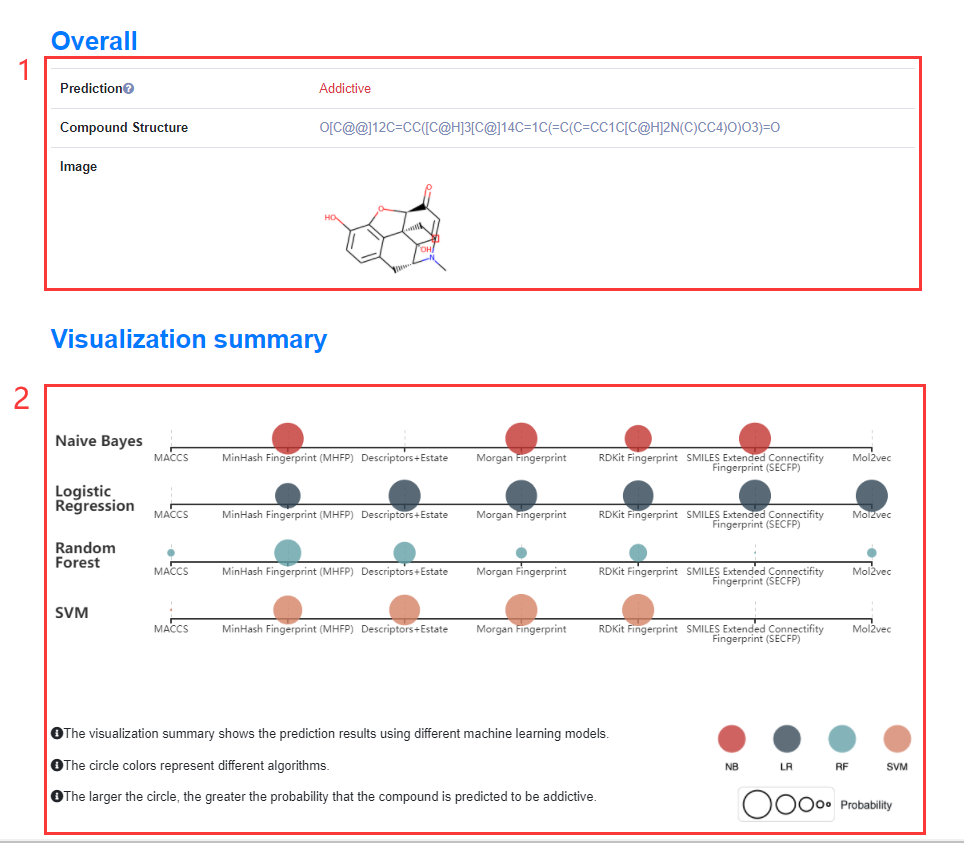
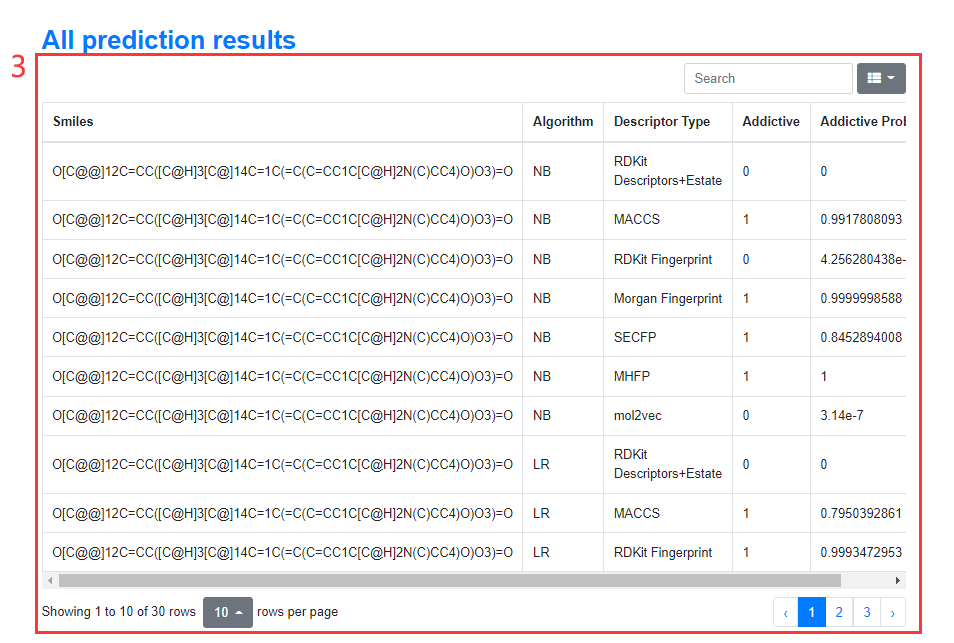
Figure 10. The results will be displayed in three parts. The first part presents the results of the consensus model, the second part visualizes the results of 29 models based on 7 descriptors for users to intuitively view, and the third part displays the specific prediction result of the above 29 models in the form of a list.
Chemical Input Tool
1. JSME
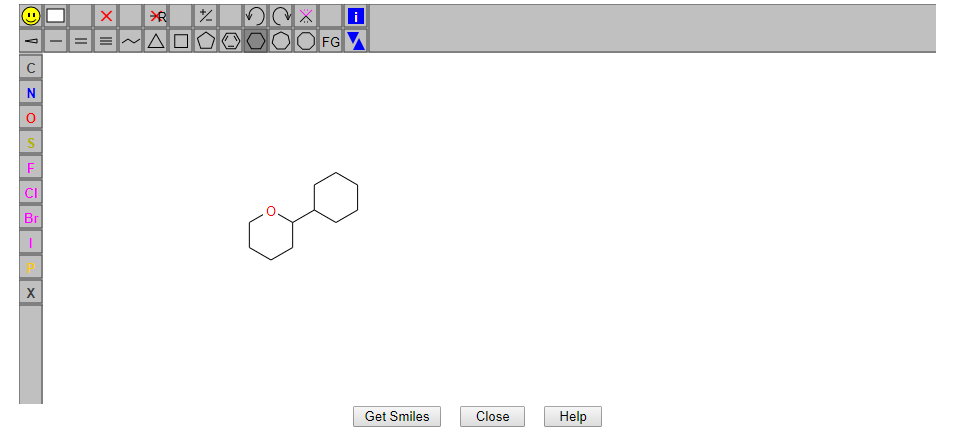
Figure 11. This JSME tool automatically converts the 2D molecular image into the SMILES format of the compound. The similarity retrieval method is based on the similarity algorithm.